Scientific Publications
Psychometric performance of a new condition-specific preference-weighted measure, VILL-UI, and EQ-5D-5L in patients with age-related macular degeneration: A MACUSTAR Study Report
26.4.2025, Value in Health, https://doi.org/10.1016/j.jval.2025.04.2155
Read the full article here
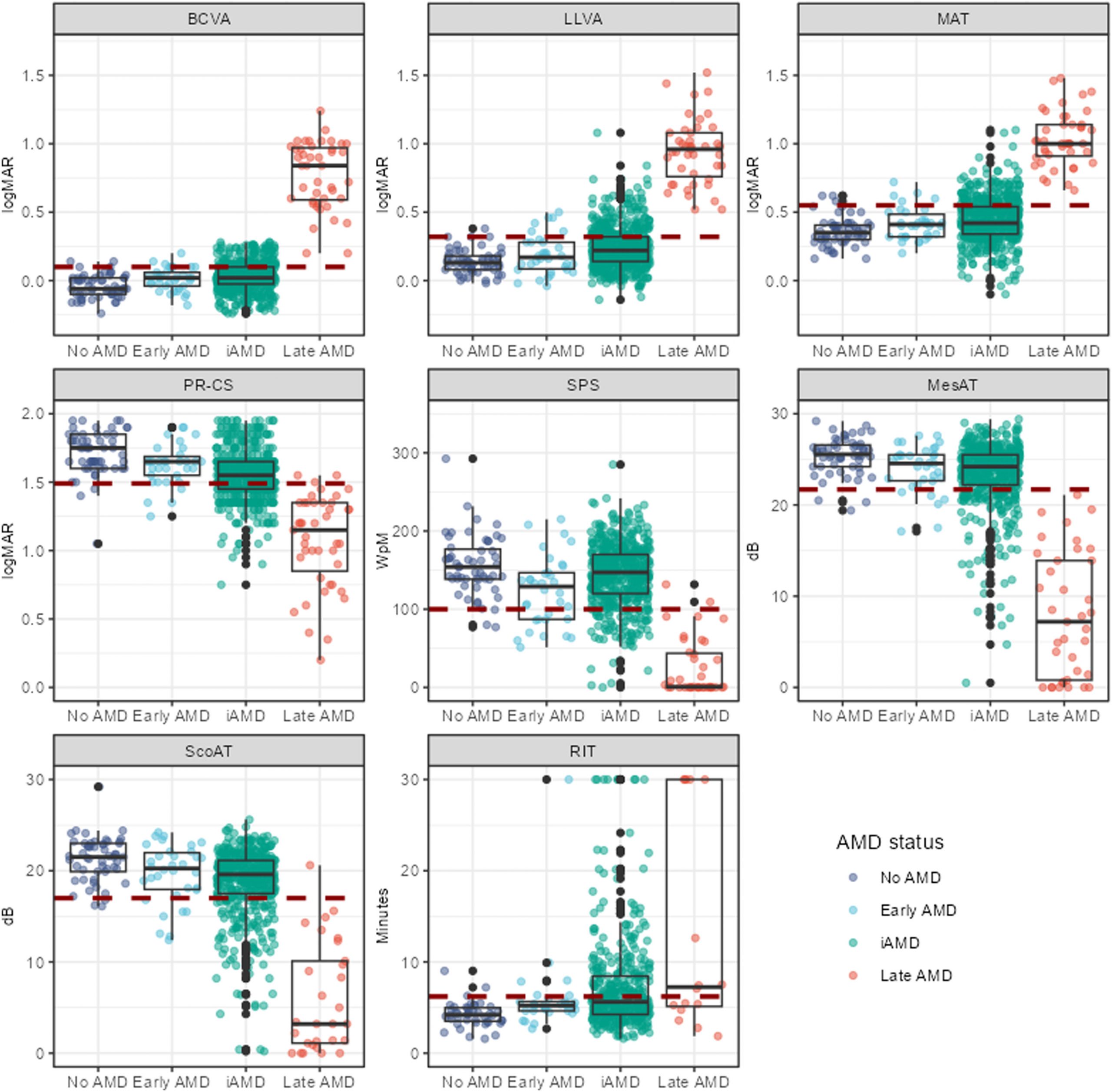
Heterogeneous Visual Function Deficits in Intermediate Age-Related Macular Degeneration: A MACUSTAR Report
Ophthalmology Science, Volume 5, Issue 4, July–August 2025, doi: 10.1016/j.xops.2025.100708.
The Spectrum of Functional, Structural, and Patient-Reported Outcomes in Intermediate Age-Related Macular Degeneration: A MACUSTAR Study Report
Genetic Risk of Reticular Pseudodrusen in Age-Related Macular Degeneration: HTRA1 /lncRNA BX842242.1 dominates, with no evidence for Complement Cascade involvement
Generalizable Deep Learning for the Detection of Incomplete and Complete Retinal Pigment Epithelium and Outer Retinal Atrophy: A MACUSTAR Report
Translational Vision Science and Technology. 2024 Sep 5;13(9):11. doi: 10.1167/tvst.13.9.11
Development and valuation of a preference-weighted measure in Age-Related Macular Degeneration from the Vision Impairment in Low Luminance (VILL) questionnaire – A MACUSTAR report
Retest variability and patient reliability indices of quantitative fundus autofluorescence in age-related macular degeneration: a MACUSTAR study report
Sci Rep. 2023 Oct. doi: 10.1038/s41598-023-43417-y.
https://www.nature.com/articles/s41598-023-43417-y
Disease-specific assessment of Vision Impairment in Low Luminance in age-related macular degeneration – a MACUSTAR study report
Br J Ophthalmology. 2023 Aug. doi: 10.1136/bjophthalmol-2021-320848. Epub 2022 Mar 30.
https://bjo.bmj.com/content/107/8/1144.long
Test-Retest Variability and Discriminatory Power of Measurements From Microperimetry and Dark Adaptation Assessment in People With Intermediate Age-Related Macular Degeneration – A MACUSTAR Study Report
Transl Vis Sci Technol. 2023 Jul. doi: 10.1167/tvst.12.7.19.
https://tvst.arvojournals.org/article.aspx?articleid=2791336
Characteristics and Spatial Distribution of Structural Features in Age-Related Macular Degeneration: A MACUSTAR Study Report
Marlene Saßmannshausen, Charlotte Behning, Jonas Weinz, Lukas Goerdt, Jan H Terheyden , Petrus Chang, Matthias Schmid, Stephen H Poor, Nadia Zakaria, Robert P Finger, Frank G Holz , Maximilian Pfau, Steffen Schmitz-Valckenberg, Sarah Thiele; MACUSTAR Consortium Members
Ophthalmol Retina. 2023 May. doi: 10.1016/j.oret.2022.12.007. Epub 2022 Dec 20.
https://www.sciencedirect.com/science/article/pii/S2468653022006091?via%3Dihub
Comparability of automated drusen volume measurements in age-related macular degeneration: a MACUSTAR study report
Repeatability and Discriminatory Power of Chart-Based Visual Function Tests in Individuals With Age-Related Macular Degeneration
A MACUSTAR Study Report
Dunbar H, Behning C, Abdirahman A, Higgins BE, Binns AM, Terheyden JH, Zakaria N, Poor S, Finger RP, Leal S, Holz FG, Schmid M, Crabb DP, Rubin GS, Luhmann UFO; for the MACUSTAR Consortium
Rubin GS, Luhmann UFO; for the MACUSTAR Consortium
Disease-specific assessment of Vision Impairment in Low Luminance in age- related macular degeneration – a MACUSTAR study report
Terheyden JH, Pondorfer SG, Behning C, Berger M, Carlton J, Rowen D, Bouchet C, Poor S, Luhmann UFO, Leal S, Holz FG, Butt T, Brazier JE, Finger RP, the MACUSTAR Consortium
Intersession Repeatability of Structural Biomarkers in Early and Intermediate Age-Related Macular Degeneration: A MACUSTAR Study Report
Saßmannshausen M, Thiele S, Behning C, Pfau M, Schmid M, Leal S, Luhmann UFO, Finger RP, Holz FG, Schmitz-Valckenberg S, on behalf of the MACUSTAR Consortium
Optimising assessment of dark adaptation data using time to event analysis

Higgins BE, Montesano G, Binns AM, Crabb DP
Scientific Reports 2021 April 15: 11. DOI: 10.1038/s41598-021-86193-3
A New Method for Visualizing Drusen and Their Progression in Flood-Illumination Adaptive Optics Ophthalmoscopy
Rossi EA, Norberg N, Eandi C, Chaumette C, Kapoor S, Le L, Snyder VC, Martel JN, Gautier J, Gocho K, Dansingani KK, Chhablani J, Arleo A, Mrejen S, Sahel J, Grieve K, Paques M
Challenges, facilitators and barriers to screening study participants in early disease stages-experience from the MACUSTAR study

Terheyden JH, Behning C, Lüning A, Wintergerst L, Basile PG, Tavares D, Melício BA, Leal S, Weissgerber G,Luhmann U, Crabb DP, Tufail A, Hoyng C, Berger M, Schmid M, Silva R, Martinho CV, Cunha-Vaz J, Holz FG and Finger RP, on behalf of the MACUSTAR consortium
BMC Medical Research Methodology 2021 March 17: 54. DOI: 10.1186/s12874-021-01243-8
Clinical Perspectives and Trends: Microperimetry as a Trial Endpoint in Retinal Disease

Yang Y, Dunbar H
Ophthalmologica 2021 February: 244. DOI: 10.1159/000515148
Structure–Function Analysis in Macular Drusen With Mesopic and Scotopic Microperimetry

Montesano G, Ometto G, Higgins BE, Iester C, Balaskas K, Tufail A, Chakravarthy U, Hogg RE, Crabb DP
TVST 2020 December: 9;13. DOI: 10.1167/tvst.9.13.43
Use of Composite End Points in Early and Intermediate Age-Related Macular Degeneration Clinical Trials: State-of-the-Art and Future Directions

Terheyden JH, Schmitz-Valckenberg S, Crabb DP, Dunbar H, Luhmann UFO, Behning C, Schmid M, Rubin GS, Silva R, Cunha-Vaz J, Tufail A, Weissgerber G, Leal S, Holz FG, Finger RP, on behalf of the MACUSTAR consortium
Ophthalmologica 2020 December 3: 11. DOI: 10.1159/000513591
Clinical study protocol for a low-interventional study in intermediate age-related macular degeneration developing novel clinical endpoints for interventional clinical trials with a regulatory and patient access intention—MACUSTAR.

Terheyden JH, Holz FG, Schmitz-Valckenberg S, Lüning A, Schmid M, Rubin GS, Dunbar H, Tufail A, Crabb DP, Binns A, Sánchez CI, Hoyng C, Margaron P, Zakaria N, Durbin M, Luhmann U, Zamiri P, Cunha-Vaz J, Martinho C, Leal S, Finger RP, MACUSTAR consortium
Trials 2020 July 18:p. 659. DOI: 10.1186/s13063-020-04595-6.
MACUSTAR: Entwicklung und klinische Validierung von funktionellen, strukturellen und patientenberichteten Endpunkten bei intermediärer altersabhängiger Makuladegeneration.

Terheyden JH, Finger RP, Schmitz-Valckenberg S, Agostini H, Dahlke C, Kuehlewein L, Lang GE, Pauleikhoff D, Wolf A, Boettger MK, Luhmann UFO, Asmus F, Holz FG, MACUSTAR consortium
Ophthalmologe 2019 May 13:1-8 DOI: 10.1007/s00347-019-0907-1
European Network of Clinical Research in Ophthalmology Information Update – December 2018.
 Martinho C, Sanches D
Martinho C, Sanches D
Ophthalmic Res 2019;28:1-5. DOI: 10.1159/000496497.
MACUSTAR: Development and Clinical Validation of Functional, Structural, and Patient-Reported Endpoints in Intermediate Age-Related Macular Degeneration.
 Finger RP, Schmitz-Valckenberg S, Schmid M, Rubin GS, Dunbar H, Tufail A, Crabb DP, Binns A, Sánchez CI, Margaron P, Normand G, Durbin MK, Luhmann UFO, Zamiri P, Cunha-Vaz J, Asmus F, Holz FG; on behalf of the MACUSTAR consortium
Finger RP, Schmitz-Valckenberg S, Schmid M, Rubin GS, Dunbar H, Tufail A, Crabb DP, Binns A, Sánchez CI, Margaron P, Normand G, Durbin MK, Luhmann UFO, Zamiri P, Cunha-Vaz J, Asmus F, Holz FG; on behalf of the MACUSTAR consortium
Ophthalmologica. 2018 Aug 28:1-12. doi: 10.1159/000491402.
European Network of Clinical Research in Ophthalmology.
Information Update – June 2017
 Martinho C, Sanches D
Martinho C, Sanches D