News
EMA endorses the continuation of MACUSTAR project with its 3rd letter of support
The Letter of Support was issued following MACUSTAR consortium requesting the European Medicine Agency´s (EMA) Scientific advice on research results from analysis of the MACUSTAR 3-year follow-up data. EMA agreed with supporting the relevance of both structural and functional assessments in intermediate AMD which could both become useful as clinical trial enrichment criteria in the future. Furthermore, it was supportive of further use of the VILL questionnaire in future AMD trials. The EMA concludes with it endorsing the goals and further research of MACUSTAR consortium to develop trial enrichment criteria and trial endpoints for subjects with iAMD.
This once again honours the excellent work done by MACUSTAR researchers, and we are happy that this is going on!
Read the full letter here.
Successful MACUSTAR General Assembly Meeting 2024 in Bonn
Members of MACUSTAR Management Board from 5 countries came together for two successful meeting days in Bonn, Germany
Photo by © Volker Lannert
New publication: MACUSTAR cohort helped better define the risk for reticular pseudodrusen in AMD
MACUSTAR imaging and genetic data helped better define the genetic risk for reticular pseudodrusen. This work by the Reticular Pseudodrusen Consortium was pre-published on 28 September 2024, while it is under final review for publication in a peer-reviewed journal.
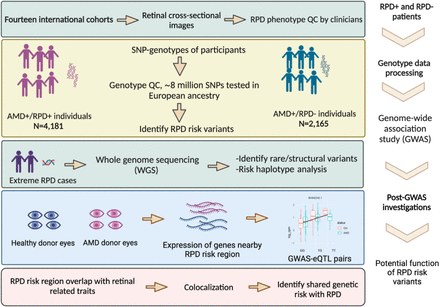
Overall study design in multiple stepwise strategies from cohort collection. MACUSTAR cohort was one of the14 cohorts analysed in this work
19 September 2024: Successful 8th MACUSTAR Investigator Meeting

From left to right: Lourdes Vidal, Rita Ribeiro (AIBILI), Angela Carneiro (CHU Porto), Ana Rita Santos (AIBILI), Charlotte Behning (UKB), Alexandra Miliu (UKB), Emanuele Fusi (HSR), Tunde Peto (QUB), Robert Finger (UKB), Frank Holz (UKB), Steffen Schmitz-Valckenberg (UKB), Jan Terheyden (UKB), Adnan Tufail (MRBC), Sergio Leal (Bayer), Rufinho Silva (AIBILI), Camiel Boon (LUMC)
19-22 September 2024: We’ll be sharing our latest MACUSTAR findings at EURETINA.
📊 Join us for exciting insights and cutting-edge developments.
September 2024: AI model for AMD biomarker detection developed using MACUSTAR data
Different researchers from MACUSTAR consortium developed a deep learning algorithm for detecting and quantifying incomplete retinal pigment epithelium and outer retinal atrophy (iRORA) and complete retinal pigment epithelium and outer retinal atrophy (cRORA) in OCT images. They then validated this model on the MACUSTAR data. Congratulations for this important work!
May 30, 2024: EMA issues a Follow up Qualification Advice letter for MACUSTAR
A regulatory interaction on the longitudinal part of the study (3-year follow-up data) was submitted in November 2023, requesting EMA‘s Scientific Advice. In response to the consortium‘s briefing document, EMA addressed several questions to MACUSTAR consortium in February 2024. Main discussion topics were potential treatment indication in iAMD and study enrichment criteria.
A fruitful discussion between EMA and MACUSTAR consortium took place on April 8 2024, addressing all issues and receiving valuable and positive feedback from EMA.
In response to the discussion, MACUSTAR consortium received a Qualification Advice Letter from EMA, which was adopted by CHMP on 30 May 2024. The Advice letter supported the relevance of both structural and functional assessments in intermediate AMD which could both become useful as clinical trial enrichment criteria in the future. Furthermore, it was supportive of further use of the VILL questionnaire in future AMD trials. A third letter of support is expected to be published by EMA.
May 5-9, 2024: MACUSTAR @ ARVO 2024 in Seattle
We are excited to present the latest findings from MACUSTAR study at ARVO 2024, from the fields of functional, structural and PRO outcomes. Do not miss the MACUSTAR paper and poster presentations during ARVO, all listed below:
February 29, 2024
MACUSTAR IMI2 funding is coming to an end, but MACUSTAR project is going to continue thanks to an extension funded by Roche, Novartis and Bayer. This extension until 2027 will permit a total of 15 follow-up visits per patient. These 6-year longitudinal data will permit the MACUSTAR consortium to better understand iAMD and to propose new biomarkers to regulatory authorities.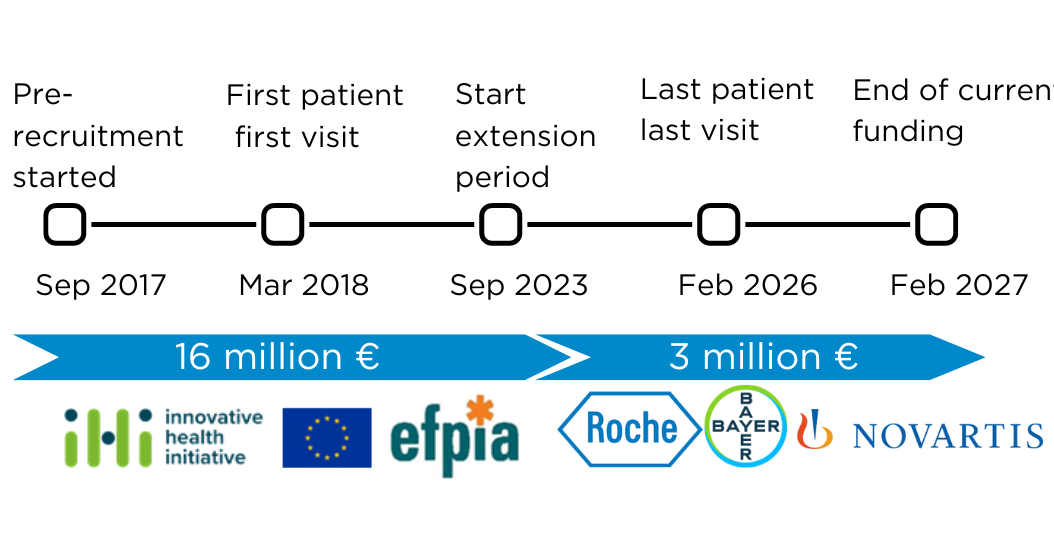
November 22-23, 2023
This year´s 7th MACUSTAR General Assembly meeting was held in Bonn from 22 to 23 November. We had very fruitful discussions on 8 different presentations covering the different MACUSTAR workgroups- so much collaborative high quality work going on!
October 23, 2023
The next EMA interaction has started: after months of preparation, we submitted our next briefing book to EMA for their feedback on our evaluation of potential clinical endpoints for iAMD.
October 13, 2023
A new publication from the MACUSTAR consortium in nature scientific reports is out: Retest variability and patient reliability indices of quantitative fundus autofluorescence in age-related macular degeneration: a MACUSTAR study report.
From the transversal part of the MACUSTAR study data, the authors analysed if the quantitative fundus autofluorescence (QAF) imaging results where consistent between the two measurements at 2 weeks average interval of the same patient. They conclude that QAF is a reliable testing of autofluorescence levels at the posterior pole in patients with AMD in a multicenter, multioperator setting.
Publication: Leon von der Emde, Merten Mallwitz, Marc Vaisband, Jan Hasenauer, Marlene Saßmannshausen, Jan Henrik Terheyden, MACUSTAR Consortium, Kenneth R. Sloan, Steffen Schmitz-Valckenberg, Robert P. Finger, Frank G. Holz & Thomas Ach. DOI: https://doi.org/10.1038/s41598-023-43417-y
October 05 – 08, 2023
MACUSTAR study was present at EURETINA 2023 in Amsterdam. Different members of the MACUSTAR consortium presented their analyses on different results done with different measurement techniques in the MACUSTAR study:
Charlotte Behning´s presentation addressed the question of which visual function metrics measured in the MACUSTAR study can best help distinguish a healthy aging eye from an iAMD eye “Discriminating intermediate AMD from normal aging changes using multiple visual function tests: A MACUSTAR study report“.
Jan Terheyden exhibited a separate set of data collected in the MACUSTAR study: Patient-reported outcomes (PRO), meaning the way the MACUSTAR patients evaluate and describe their own visual abilities in everyday situations as well as their general health and life quality. Jan analysed how these PRO relate to the health of the eye as determined by imaging techniques. Find the abstract for Jan´s poster here: Patient-reported vision impairment in low luminance and general health domains in intermediate age-related macular degeneration: A MACUSTAR study report.
Aside from that, Philippe Valmaggia explored the MACUSTAR microperimetry data. He presented a model that aims to describe how mesopic and photopic sensitivity changes across different AMD stages in Retinotopic sensitivity prediction in age-related macular degeneration based on optical coherence tomography data.
EURETINA Amsterdam was the perfect setting to hold MACUSTAR investigators meeting 2023. This investigators meeting 2023 was a resounding success: it was well attended, both from clinical sites staff and members of the management board. The feedback received from the clinical sites was very positive, the reduction of exams to be performed and reduction of patients’ burden was appreciated for the study´s extension period.
November 25-26, 2022
After two years of virtual meeting due to COVID-19 restrictions, this year´s MACUSTAR Management Board took place face-to-face in Bonn, Germany. Members discussed the progress of the study, recent results, and the sustainability of the MACUSTAR project beyond the IMI funding period.
September 2, 2022
The lecture “MACUSTAR – An ongoing European Union IMI2 study on intermediate AMD clinical outcome parameter” was held by Prof. Frank Holz at the annual meeting of the European society of retina specialists (EURETINA) in Hamburg, Germany. During the symposium of the European Vision Clinical Research network (EVICR.net), the MACUSTAR study was intensively discussed among other innovative studies in the field.
June 27, 2022
This year’s meeting of the MACUSTAR Scientific Advisory Board (SAB) took place as a virtual meeting. More than 25 participants from the SAB and the MACUSTAR consortium attended the meeting. Topics around the results of the cross-sectional Clinical Study as well as recent scientific achievements and updates were discussed intensively.
June 27, 2022
MACUSTAR Study extended until February 2024: During the second scientific advice procedure EMA proposed an extension of the MACUSTAR study. The project has now been officially extended by 18 months.
June 23, 2022
New publication from the MACUSTAR consortium in JAMA Ophthalmology: Repeatability and Discriminatory Power of Chart-Based Visual Function Tests in Individuals With Age-Related Macular Degeneration – A MACUSTAR Study Report
There is a need for validated clinical end points that are reliably able to quantify potential therapeutic effects of future treatments targeting age-related macular degeneration (AMD) before the onset of serious visual impairment. Under multicenter, multiexaminer conditions, do simple chart-based assessments of visual function (VF) have sufficient repeatability and discrimination in people with age-related macular degeneration (AMD) to be considered as measures for future clinical trial end points? In this cross-sectional study including 245 people with AMD and 56 healthy, age-similar control individuals, best-corrected visual acuity, low-luminance visual acuity, Moorfields Acuity Test, contrast sensitivity, and International Reading Speed Test had adequate repeatability but limited power to discriminate between no AMD and intermediate AMD (iAMD). The findings suggest that the chart-based tests included in this study perform sufficiently well to be considered as potential measures for clinical trial end points; their prognostic power to predict conversion from iAMD to late AMD needs to be examined with longitudinal data.
Publication: Hannah M. P. Dunbar, Charlotte Behning, Amina Abdirahman, Bethany E. Higgins, Alison M. Binns, Jan H. Terheyden, Nadia Zakaria, Stephen Poor, Robert P. Finger, Sergio Leal, Frank G. Holz, Matthias Schmid, David P. Crabb, Gary S. Rubin, Ulrich F. O. Luhmann, for the MACUSTAR Consortium, doi:10.1001/jamaophthalmol.2022.2113
May 01-04, 2022
Results of MACUSTAR cross-sectional study presented at ARVO 2022 in Denver:
- Comparison of visual function in structurally defined sub-phenotypes of intermediate AMD: A MACUSTAR study report.
by Hannah M P Dunbar; Marlene Sassmannshausen; Charlotte Behning; Sarah Thiele; Alison Binns; Bethany Elora Higgins; Jan Henrik Terheyden; Adnan Tufail; Sergio Leal; Nadia Zakaria; Frank G Holz; Matthias C Schmid; Robert P Finger; David P. Crabb; Steffen Schmitz-Valckenberg; Ulrich F O Luhmann
Though ascribed the same AMD disease stage, eyes with iAMD+RPD and iAMD+cRORA had poorer low luminance and contrast vision than other iAMD eyes. As the number of eyes with observed structural features is small, we aim to confirm our findings in a large longitudinal cohort and examine whether additional structural or functional features should be accounted for in AMD disease classification or used as entry criteria in treatment trials.
- The impact of illumination and disease stage on navigation performance in age-related macular degeneration (AMD): A MACUSTAR study report.
by Ariel Zenouda; Alina Schenk; Amina Abdirahman; Charlotte Behning; José-Alain Sahel; Adnan Tufail; Nadia Zakaria; Nick Tyler; Saddek Mohand-Said; Michel Paques; Ulrich F O Luhmann; Hannah M P Dunbar
A navigation task was developed to study the impact of illumination and disease stage on navigation performance in people with AMD. Results indicate that whilst those with late AMD have poorer navigation performance under low and transient light conditions, people with e/i AMD do not experience a deleterious effect of static low light, but are negatively impacted by a sudden reduction in luminance. This may be a real-world manifestation of delayed dark adaptation and impaired vision in low luminance. These findings support the exploration of the utility of navigation-based endpoints for future novel treatment trials in both e/i and late AMD.
- Inter-device and inter-scan comparability of drusen volume assessments in age-related macular degeneration: A MACUSTAR report.
by Davide Garzone; Robert P Finger; Olivier Morelle; Maximilian W M Wintergerst; Marlene Sassmannshausen; Steffen Schmitz-Valckenberg; Maximilian Pfau; Sarah Thiele; Stephen Poor; Sergio Leal; Frank G Holz; Jan Henrik Terheyden
Optical coherence tomography (OCT)-based drusen volume quantification is a relevant biomarker in age-related macular degeneration (AMD). An FDA-approved automated software for quantifying drusen volume using Cirrus (Zeiss Meditec, Dublin, CA) is available, however the comparability with similar approaches on other devices is largely unknown. Hence, we compared drusen volume measured by a newly developed, automated software running on Spectralis OCT data (Heidelberg Engineering, Heidelberg, Germany) against the above-mentioned software on Cirrus.
March 30, 2022
New publication from the MACUSTAR consortium in British Journal of Ophthalmology: Disease-specific assessment of Vision Impairment in Low Luminance in age- related macular degeneration – a MACUSTAR study report
Patient relevance is key for regulatory assessment of age-related macular degeneration (AMD) treatments, but existing patient-reported outcome instruments do not fulfil development requirements by regulators or capture AMD patients’ difficulties insufficiently. The Vision Impairment in Low Luminance (VILL) questionnaire has been developed according to regulatory guidelines and is implemented in the MACUSTAR study. This study supports the psychometric performance including internal consistency, item fit, subscale structure, test–retest reliability and construct validity of the VILL in a multinational, multilanguage setting. The study supports that the VILL is sufficiently precise to capture patient-reported deficits in
AMD in future trials
Publication: Jan Henrik Terheyden, Susanne G Pondorfer, Charlotte Behning, Moritz Berger, Jill Carlton, Donna Rowen, Christine Bouchet, Stephen Poor, Ulrich F O Luhmann, Sergio Leal, Frank G Holz, Thomas Butt, John E Brazier, Robert P Finger, the MACUSTAR consortium, doi: 10.1136/bjophthalmol-2021-320848
March 25, 2022
New publication from the MACUSTAR consortium in Translational Vision Science & Technology: Intersession Repeatability of Structural Biomarkers in Early and Intermediate Age-Related Macular Degeneration: A MACUSTAR Study Report
Drusen are dynamic sub-RPE deposits that are risk factors for late-stage age-related macular degeneration (AMD). We show a new method using flood-illumination adaptive optics (FIAO) that reveal drusen with high contrast and resolution.
This new technique offers promise as a robust and sensitive method to detect, map, quantify, and monitor the dynamics of drusen in aging and AMD.
Publication: Marlene Saßmannshausen, Sarah Thiele, Charlotte Behning, Maximilian Pfau, Matthias Schmid, Sérgio Leal, Ulrich F. O. Luhmann, Robert P. Finger, Frank G. Holz, and Steffen Schmitz-Valckenberg, on behalf of the MACUSTAR Consortium, https://doi.org/10.1167/tvst.11.3.27
Feb 15, 2022
European medicines agency (EMA) supports the further conduct of the MACUSTAR clinical study to develop functional and/or structural biomarkers as possible future primary endpoints for clinical trials after reviewing the results of the cross-sectional study MACUSTAR receives second Letter of Support from EMA.
December 17, 2021
New publication from the MACUSTAR consortium in Translational Vision Science & Technology: A New Method for Visualizing Drusen and Their Progression in Flood-Illumination Adaptive Optics Ophthalmoscopy
Recruiting asymptomatic participants with early disease stages into studies is challenging and only little is known about facilitators and barriers to screening and recruitment of study participants. Thus we assessed factors associated with screening rates in the MACUSTAR study, a multi-centre, low-interventional cohort study of early stages of age-related macular degeneration (AMD). Screening rates per clinical site and per week were compiled and applicable recruitment factors were assigned to respective time periods. A generalized linear mixed-effects model including the most relevant recruitment factors identified via in-depth interviews with study personnel was fitted to the screening data.
Publication: Ethan A. Rossi, Nathaniel Norberg, Chiara Eandi, Celine Chaumette, Saloni Kapoor, Laura Le, Valerie C. Snyder, Joseph N. Martel, Josselin Gautier, Kiyoko Gocho, Kunal K. Dansingani, Jay Chhablani, Angelo Arleo, Sarah Mrejen, José-Alain Sahel, Kate Grieve, Michel Paques, https://doi.org/10.1167/tvst.10.14.19
October 29, 2021
The annual meeting of the MACUSTAR Management Board took place as a virtual meeting. Members from all over Europe attended the discussions on current topics and the status of the Clinical Study. Also this year the participation of the consortium members was very high.
September 09-12, 2021
The team of the MACUSTAR project attended EURETINA 2021 Virtual, showing results of the cross-sectional study. Robert Finger also presented the latest developments of the MACUSTAR study in the symposium of the European Vision Clinical Research Network (EVICR.NET), “Macustar – An ongoing IMI2 study on AMD outcomes“.
March 17, 2021
New publication from the MACUSTAR consortium in BMC Medical Research Methodology: Challenges, facilitators and barriers to screening study participants in early disease stages-experience from the MACUSTAR study
Recruiting asymptomatic participants with early disease stages into studies is challenging and only little is known about facilitators and barriers to screening and recruitment of study participants. Thus we assessed factors associated with screening rates in the MACUSTAR study, a multi-centre, low-interventional cohort study of early stages of age-related macular degeneration (AMD). Screening rates per clinical site and per week were compiled and applicable recruitment factors were assigned to respective time periods. A generalized linear mixed-effects model including the most relevant recruitment factors identified via in-depth interviews with study personnel was fitted to the screening data.
Publication: Jan H. Terheyden, Charlotte Behning, Anna Lüning, Ludmila Wintergerst, Pier G. Basile, Diana Tavares, Beatriz A. Melício, Sergio Leal, George Weissgerber, Ulrich F. O. Luhmann, David P. Crabb, Adnan Tufail, Carel Hoyng, Moritz Berger, Matthias Schmid, Rufino Silva, Cecília V. Martinho, José Cunha-Vaz, Frank G. Holz and Robert P. Finger, on behalf of the MACUSTAR consortium, Challenges, facilitators and barriers to screening study participants in early disease stages-experience from the MACUSTAR study, BMC Medical Research Methodology, https://doi.org/10.1186/s12874-021-01243-8
December 07, 2020
New review article from the MACUSTAR consortium in Ophthalmologica: Use of composite endpoints in early and intermediate age-related macular degeneration clinical trials – state-of-the-art and future directions
The slow progression of early AMD stages to advanced AMD requires the use of surrogate endpoints in clinical trials. The use of combined endpoints may allow for shorter and smaller trials due to increased precision. The review article gives an overview of the use of composite endpoints as primary outcome measures in clinical studies of early AMD stages. After reviewing a total of 673 abstracts and applicable full-text articles, 33 articles were eligible and thus were included in the qualitative synthesis. The main composite endpoint categories were: Combined structural and functional endpoints, combined structural endpoints, combined functional endpoints and combined multi-categorical endpoints. The majority of the studies included binary composite endpoints. There was a lack of sensitivity analyses of different endpoints against accepted outcomes (i.e. progression) in the literature. Various composite outcome measures have been used but there is a lack of standardization. To date no agreement on the optimal approach to implement combined endpoints in clinical studies of early stages of AMD exists and no surrogate endpoints have been accepted for AMD progression.
Publication: Jan H. Terheyden, Steffen Schmitz-Valckenberg, David P. Crabb, Hannah Dunbar, Ulrich F. O. Luhmann, Charlotte Behning, Matthias Schmid, Rufino Silva, José Cunha-Vaz, Adnan Tufail, George Weissgerber, Sergio Leal, Frank G. Holz, Robert P. Finger, Use of composite endpoints in early and intermediate age-related macular degeneration clinical trials – state-of-the-art and future directions, Ophthalmologica, https://doi.org/10.1159/000513591
November 27, 2020
The annual meeting of the MACUSTAR Management Board took place as a virtual meeting. Members from all over Europe attended the discussion on the current progress of the Clinical Study as well as current topics. 40 members of the consortium and the IMI were present at the three-hour meeting.
July 18, 2020
New publication from the MACUSTAR consortium in Trials: Clinical study protocol for a low-interventional study in intermediate age-related macular degeneration developing novel clinical endpoints for interventional clinical trials with a regulatory and patient access intention—MACUSTAR
The protocol published in the peer-reviewed journal Trials describes the low-interventional clinical multicenter study MACUSTAR employing a novel two-part design. The cross-sectional part (total duration, 1 month) and the longitudinal part (total duration, 36 months) include participants with iAMD and control groups with early/late/no AMD. The cross-sectional part’s primary objective is a technical evaluation of functional, structural, and patient-reported outcomes. The longitudinal part’s primary objective is to assess the prognostic significance in functional, structural, and patient-reported outcomes for the progression from iAMD to late AMD. All data will be used to support a biomarker qualification procedure by regulatory authorities.
Publication: Jan H. Terheyden, Frank G. Holz, Steffen Schmitz-Valckenberg, Anna Lüning, Matthias Schmid, Gary S. Rubin, Hannah Dunbar, Adnan Tufail, David P. Crabb, Alison Binns, Clara I. Sánchez, Carel Hoyng, Philippe Margaron, Nadia Zakaria, Mary Durbin, Ulrich Luhmann, Parisa Zamiri, José Cunha-Vaz, Cecília Martinho, Sergio Leal, Robert P. Finger: Clinical study protocol for a low-interventional study in intermediate age-related macular degeneration developing novel clinical endpoints for interventional clinical trials with a regulatory and patient access intention—MACUSTAR, Trials, https://doi.org/10.1186/s13063-020-04595-6
March 23, 2020
The MACUSTAR project has completed recruitment of over 700 individuals with age-related macular degeneration for the clinical study on the development of novel endpoints for intermediate age-related macular degeneration (iAMD). A total of 20 designated clinical sites in Europe participated and enrolled 585 iAMD patients and over 130 age-matched control subjects without AMD, with early and late AMD. A review period of up to three years will assess disease progression and risk factors with a series of functional tests, imaging modalities and patient-reported outcomes. Novel functional tests for the specific visual deficit in iAMD include dark-adapted microperimetry and standardized dark adaptation, as well as, state-of-the-art high-resolution retinal imaging including optical coherence tomography (OCT) and OCT angiography.
November 15-16, 2019
The annual meeting of the MACUSTAR Management Board took place in Berlin, Germany. Members from all over Europe discussed topics around the Clinical Study as well as recent advances of the work packages. In addition, the recruitment period was adjusted following a review of the recruitment status. A representative of the IMI participated in the event.

September 6, 2019
The lecture “MACUSTAR – An IMI2 study on AMD outcomes” held by Prof. Frank Holz at the annual meeting of the European society of retina specialists (EURETINA) in Paris, France was met with great interest. The symposium of the European Vision Clinical Research network (EVICR.net) allowed discussions on several innovative studies in the field.
April 29, 2019
This year’s meeting of the MACUSTAR Scientific Advisory Board (SAB) took place at ARVO 2019 in Vancouver, British Columbia. More than 30 participants from the SAB and the MACUSTAR consortium attended the meeting. Topics around the Clinical Study as well as structural, functional and patient-reported endpoints addressed in the MACUSTAR were discussed intensively.
April 28, 2019
From the ARVO annual meeting, 2019:
- Illumination affects mobility performance in patients with age-related macular degeneration
by Hannah Dunbar, Hannah1,2 ; Ariel Zenouda4 ; Saddek Mohand-Saïd3 ; Adnan Tufail 1,2 ; Jose A. Sahel3,4 ; Gary S. Rubin1,2 on behalf of the MACUSTAR Consortium
(1. Visual Neuroscience, UCL Institute of Ophthalmology, London, United Kingdom; 2.Moorfields Eye Hospital NHS Foundation Trust, United Kingdom; 3. INSERM-DHOS CIC 1423, CHNO des Quinze-Vingts, Paris, France; 4. Streetlab SAS, Institut de la Vision, France)
Data from ongoing MACUSTAR navigation performance assessments were reported at ARVO 2019. Researchers at University College London (UCL) and Institut de la Vision, Paris invited subjects recruited at Moorfields Eye Hospital and Centre National d’Ophtalmologie des Quinze-Vingts respectively, to take part in a navigation performance test. Subjects were asked to navigate 4 obstacle seeded mazes under 4 different light conditions: 3 static conditions (256, 4, 1 lux) and 1 transition condition (256 reducing to 1 lux) at their fastest comfortable pace whilst avoiding obstacles: 3 normal controls and 28 subjects with age-related macular degeneration took part. Light level had a significant impact on walking speed and number of navigation errors (collisions or disorientation), with significantly slower walking speeds in the transition condition as compared to the brightest condition (256 lux) and significantly more errors in the transition condition compared to all other light conditions. These results suggest subjects with AMD experience increased difficulty with navigation under a transient reducing light condition (i.e. walking from a brightly lit area to a dimly lit area), as compared to static low light conditions to which they have been adapted. This work is ongoing.
December 12, 2018
The MACUSTAR clinical study now recruits at all 20 sites.
November 9-10, 2018
 The MACUSTAR Management Board met in Basel, Switzerland to discuss the current progress of the study. In a round table discussion as well as in smaller working groups, current topics were discussed and the recent advances of the work packages were presented. More than 40 members of the consortium and the IMI from all over Europe were present at our meeting.
The MACUSTAR Management Board met in Basel, Switzerland to discuss the current progress of the study. In a round table discussion as well as in smaller working groups, current topics were discussed and the recent advances of the work packages were presented. More than 40 members of the consortium and the IMI from all over Europe were present at our meeting.

September 23, 2018

The team of the MACUSTAR project was present at EURETINA 2018 Congress from September 20 to September 23 in Vienna, Austria. We had good and intensive discussions at the Investigator Meeting on Thursday. Later that day, Frank Holz and Robert Finger also presented the MACUSTAR study in the symposium of the European Vision Clinical Research Network (EVICR.NET), “Focus on Multinational Clinical Research”.
August 28, 2018
New publication from the macustar consortium in Ophthalmologica: Development and clinical validation of functional, structural and patientreported endpoints in intermediate age-related macular degeneration
The article published in the peer-reviewed journal Ophthalmologica summarizes the methods used in the macustar clinical study to assess AMD and its impact on function and quality of life. For example, high-resolution imaging techniques will provide information on anatomical changes in the retina. Besides conventional visual function tests, vision under low-light conditions and contrast vision will be determined. Researchers will also capture the light sensitivity of the macula, the duration of dark adaptation, and reading speed and visual path navigation under low-light conditions. In addition, questionnaires will provide information on how visual impairment is perceived by the study participants. The macustar consortium aims to identify the best method or combination of methods that indicate if a novel therapeutic approach can stop AMD progression in the future.
Publication: Robert P. Finger, Steffen Schmitz-Valckenberg, Matthias Schmid, Gary S. Rubin, Hannah Dunbar, Adnan Tufail, David P. Crabb, Alison Binns, Clara I. Sánchez, Philippe Margaron, Guillaume Normand, Mary Durbin, Ulrich F. O. Luhmann, Parisa Zamiri, José Cunha-Vaz, Friedrich Asmus, Frank G. Holz: Development and clinical validation of functional, structural and patientreported endpoints in intermediate age-related macular degeneration, Ophthalmologica, DOI: 10.1159/000491402.
May 23, 2018
From the ARVO Annual Meeting, 2018:
- Patients with AMD report difficulty with vision-related activities and functioning under visually challenging conditions at all stages of the disease in individual interviews and focus group discussions. The difficulties include reading, social interaction/recognizing people, mobility/safety and the socio-emotional impact of these difficulties.
by Robert P. Finger (University of Bonn, Bonn, Germany)
Age-relate macular degeneration (AMD) leads to vision problems in low contrast or low luminance situations. To date, it is difficult to capture these problems in a standardized way using patient-report. Thus, we interviewed patients and collected aspects of these problems important to them which broadly fall into the categories of reading, social interaction/recognizing people, mobility/safety and the socio-emotional impact of these difficulties. These aspects should be considered when developing an instrument to capture patient-reported difficulty with vision-related activities and functioning under visually challenging conditions for AMD.
May 17, 2018
From the ARVO Annual Meeting, 2018:
- Pilot study determines that a dark adaptation test location at the edge of the macular region provides good discrimination between healthy and intermediate AMD states within a clinically viable testing timeframe

by Alison M. Binns, Laura Edwards, Deanna Taylor, David Crabb (Optometry and Visual Science, City, University of London, London, United Kingdom)
A pilot study was conducted at City, University of London to determine the optimal test conditions for the longitudinal evaluation of dark adaptation in people with intermediate age-related macular degeneration (iAMD) for the MACUSTAR study. The rod intercept time was assessed using the AdaptDX device under 5 test conditions over 2 visits: 76%, 70% and 65% rhodopsin bleach at 5 degrees eccentricity and 76% and 70% bleach at 12 degrees. Fourteen people with iAMD and 10 controls attended for 2 visits. Nearly half of the participants with iAMD produced an unacceptably long recovery time (>20mins) using a 76% bleach at 5 degrees eccentricity. The 76% bleach at 12 degrees provided almost equivalent separation between iAMD and controls but recovery was achieved within 20 minutes for almost all participants. The latter condition was therefore deemed to be most suitable for this clinical application.
The work has also been published in the journal Investigative Ophthalmology and Visual Science under the DOI: 10.1167/iovs.18-24211
Mar 26, 2018
The first patient was screened for the macustar clinical study today in Coimbra, Portugal.
The macustar clinical study can be found on ClinicalTrials.gov also. Patients will be recruited until February 2019.
Feb 15, 2018
European medicines agency (EMA) supports the approach of the MACUSTAR clinical study to develop functional and/or structural biomarkers as possible future primary endpoints for clinical trials
Oct 20, 2017
macustar kick-off meeting
Bonn
Sept 8, 2017
macustar Investigators’ Meeting at EURETINA congress
Barcelona
Robert Finger, EURETINA 2017 (David Crabb, twitter, @crabb-lab)
Sept 1, 2017
Official start of the macustar project
 Kick-off: 2nd–stage Berlin 2016
Kick-off: 2nd–stage Berlin 2016










